Warning: Parameter 2 to qtranxf_postsFilter() expected to be a reference, value given in /home/parallel/public_html/wp-includes/class-wp-hook.php on line 324
Mae Gethin Sherrington yw’r pedwerydd mwyaf poblogaidd chef bobydd â hyfforddiant ffiseg yn y Gogledd Ddwyrain, ac yn uno gwyddoniaeth a choginio ar ei flog artisaniaeth.com ac ar YouTube. Mae ei ysgrifennu a’i archwiliadau yn unigryw, felly mae parallel.cymru wrth ei bodd yn cyflwyno ei waith yma.
Gethin Sherrington is the fourth-best physics trained baker-chef in all of North West Wales, and unifies science and cooking on his blog artisaniaeth.com and on YouTube. His writing and explorations is unique, so parallel.cymru is delighted to present his work here.
O blantos bach i’r Michelin chef, dyw wyau’m yn ddieithr i neb. Wyau, yw asgwrn cefn y gegin. Efallai eich bo’n mwynhau ŵy ‘di sgramblo bora ‘ma, neu’n mentro’r drewdod a ca’ brechdan ŵy i ginio. Neu efallai eich bod, fel y gorau ohonom, yn rhythu ar oergell wag, ond mai’n iawn, mae omlet, neu quiche bach, o hyd ar gael. Allwch chi ddeall felly, pam mae wyau yw’r pwnc cyntaf sydd rhaid i ni ddadansoddi, yma ar Artisaniaeth. Y cynhwysyn cymhlethaf cyffredin, yn y gegin.
Eggs. The backbone of the kitchen. Young and old, toddler to Michelin, we’re all ovivorous beings. Perhaps you’ve just had some scrambled eggs for breakfast, or braving the stink of an egg mayo sandwich for lunch. Or maybe there’s just nothing in the fridge, but it’s OK, because there’s always omelet, or a quick quiche, for dinner. It’d be appropriate then, that the first deep dive article on Atrisaniaeth, should be on eggs. They are, after all, simultaneously the most complex, and the most commonplace ingredient there is.
| Problem Dŵy-ran "You couldn't boil an egg" dywed wrth y prentis, amheuaeth o'i anallu at dasg hollol syml. Ar y llaw arall, dewin yw'r dyn all alw o'r ŵy, riw facaron neu soufflé diwall. | An Egg-stream Dichotomy "You couldn't boil an egg" , whilst, conjuring a macaron, or hot soufflé is considered wizardry. |
Any sufficiently advanced technology is indistinguishable from magic. - Arthur C. Clarke |
|
| Nid i ddweud bod wyau o hyd am fod tu hwnt i'n dealltwriaeth. Ond eu bod nhw'n gymhlethach na'r edrychom. Mor ansefydlog, ac ansicr a'n barn ni amdanyn nhw. | That isn't to say that eggs are beyond our comprehension. It simply means they're complex and bipolar by nature. |
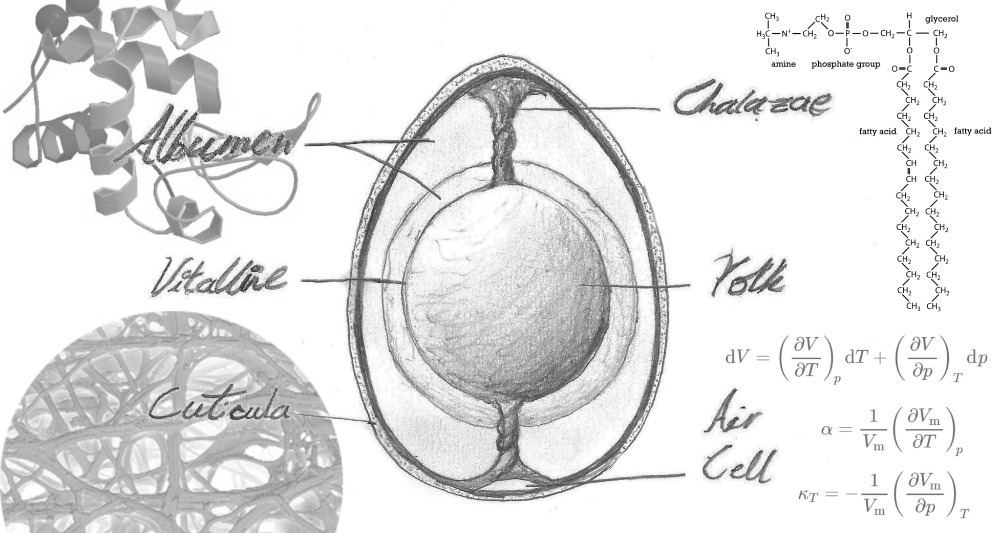
| Wedi'r cyfan, dyblyg yw natur ŵy, ei hun yn gynhwysyn o gynhwysion; o wynnwy, ac o felynwy. Tra bod y melyn efo canran cyfartal o ddŵr i fwynau, protein a braster, mae'r gwynnwy, neu albwmen, yn 90% dŵr efo'r gweddill yn brotein pur. Ond, ar y cyfan, mae'r albwmen a'r melynwy yn rhannu protein yr holl ŵy yn gyfartal rhyngddynt! | By definition, an egg is its self an ingredient of ingredients; the yolk, and the whites. Whilst yolks are a 50/50 blend of water with various fats, proteins and mineral compounds, the whites, or albumen, are almost 90% water the remainder being pure protein. However, taken as a whole, the yolk and albumen contain equal amounts of protein, sharing the egg's overall protein content 50/50. |
| Protein felly, yw gwir werth wyau yn y gegin. Dyma pam cawn wyau ym mhopeth, o gacenni a thartenni i saws stir fry. Cadwyni Carbon ydyn nhw, efo llu o grwpiau cemegol ynghlwm â nhw. Mae'r grwpiau yma'n achos y cadwyni i glymu a chysylltu efo'i gilydd, a dyma sy'n rhoi bywyd i'n bwyd, a'n bywydau. | Protein then. It's important! It's why eggs end up everywhere from cakes and tarts to soups and fry ups. They're long chains of Hydrocarbons, bonded with all sorts of functional groups. These groups are what produce such wide varieties of protein interaction, and which give life to us, and to our food. |
| Gwres: Injan ein bwyd Gwres yw'r teclyn gorau i chwarae efo protein, mesur o egni cinetig pob moleciwl mewn sylwedd. Efo mwy o wres yn cyfieithu i fwy o egni, a mwy o symudiad, yn y molecylau. | Teggnology at Work Heat, is the most basic protein manipulator, a measure of the kinetic energy possessed by all molecules within a given substance. The hotter something gets, the faster, and more energetic it's molecules become. |
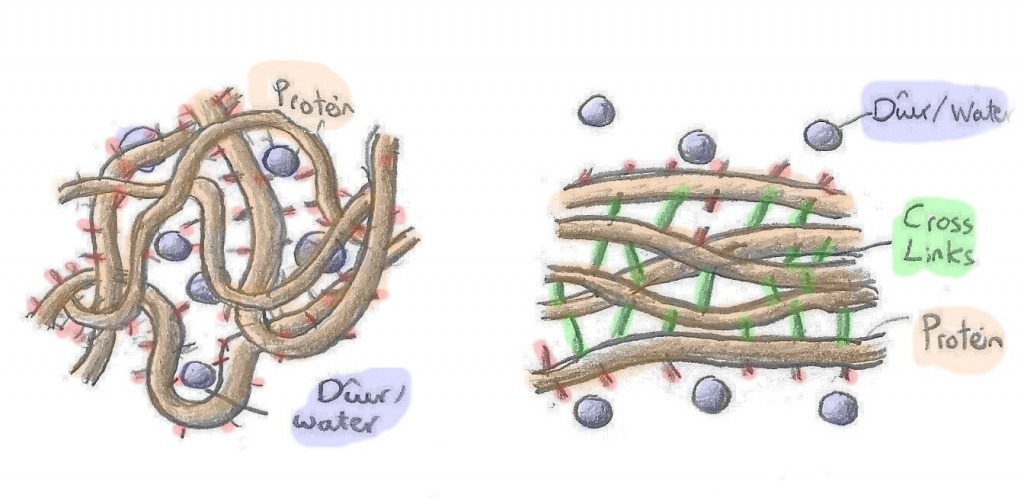
| Efo protein, gall wres achosi pob math o newidiadau. Yn gyntaf, gall strwythur y protein newid. Efo'r holl grwpiau cemegol lawr hyd enfawr y moleciwl, bydd anghyfartaledd rhwng gwefrau yn ymddangos rhwng adrannau o'r protein. Y canlyniad yw protein sy'n plygu a chorddi'r wrth i'r adrannau anghytbwys yma atynnu a gwrthyrru'i gilydd. Wrth ychwanegu gwres, gall y protein oresgyn y grymoedd electrostatig 'ma, i ddatglymu. | With regard to proteins, heat can cause many different things to occur. Firstly, the protein's structure can change. Due to their sheer size, proteins will often have unbalanced charges running along their lengths. This leads to the protein bunching and knotting its self up, as different sections of the protein are attracted and repelled by one another. Heating a protein and increasing it's energy can therefore allow the protein to overcome these forces between sections, causing it to unfurl, like a loose shoelace on a long run. |
| Wrth i'r protein agor allan, bydd y gwefrau anghytbwys yn rhydd i ymrwymo mewn ffyrdd hollol newydd, a gyda phroteinau hollol wahanol. Yn y modd yma, mae ail effaith gwres ar brotein yn ymddangos; ei allu i gynyddu cyflymder. Er mwyn cael proteinau'n adweithio, rhaid bod nhw'n cwrdd, ac er mwyn cwrdd, rhaid iddyn nhw symud...lot. | Once a protein has unfurled, the unbalanced charges along it's length will be free to bond in new ways and with other proteins. This is another affect heating has on protein. Not only does the protein have the energy to untangle its self, but it can also move around more, increasing the probability of it colliding with another unfurled protein and bonding with it. |
|
|
| Mae cynhesu protein yn arwain at broteinau yn dadrwymo, gwrthdaro a bondio, i greu rhwydwaith cadarn sy'n dal dŵr. Ceulo (coagulate) yw'r enw ar y broses hon, sy'n golygu felly mae gel yw ŵy wedi' gwcio. Gel sy'n tynhau, caledu, a cholli dŵr wrth gynhesu'n bellach. | If you imagine this scenario, you can start to see why eggs tend to solidify as they're heated, with egg proteins unfurling, colliding, and bonding as heat is applied and their energy increases. This is what leads to coagulation, where the egg sets into a semi sold structure and is no longer a liquid. |
| I wneud bywyd yn anoddach, mae'r gwahaniaeth rhwng protein y gwyn a'r melyn yn achosi i'r ddau geulo ar dymereddau hollol wahanol. Does felly'r fath beth a'r ŵy perffaith, mond riw 'sbectrwm o barodrwydd' efo ond rhannau o'r ŵy 'di gwcio'n berffaith. Cam Cogino & Tymheredd Gwynnwy yn twchu 145ºF/63ºC Ovotransferrin, sef 12% o brotein y gwyn, yn ceulo 150ºF/65ºC Melynwy yn dechrau twchu 150ºF/65ºC Melynwy yn ceulo'n llawn 158ºF/70ºC Holl wynwy'n caledu (Ovalbumin, protein pennaf y gwyn, yn ceulo) 180ºF/80ºC, | The differing protein types and concentrations in our eggs also means that there's no clear cut temperature at which an egg is 'done', instead, you have a temperature spectrum: Cooking Process & Temperature Whites thicken 145ºF/63ºC Whites become solid (ovotransferrin, 12% of total albumen protein content) 150ºF/65ºC Yolk starts to thicken 150ºF/65ºC Yolk Sets 158ºF/70ºC Whites become firm (Major albumen protein, ovalbumin, coagulates) 180ºF/80ºC, |
| Dyma pam bod rheolaeth fanwl o gwcio wyau'n agos i amhosib! Dim rhyfedd bod chwedlau a rheolau tybiedig yn rhemp trwy'n ceginau. | Given the evidence, it's no wonder why so many myths and supposed rules are circulating about eggs, and why precisely manipulating eggs, is such a challenge for cooks. |
| Mae felly angen dadansoddiad trylwyr o'r wyau 'ma'. I'w cracio ar agor, i weld yn union beth sydd yn mynd ymlaen o dan y plisgyn bach eiddil na. Ychwan-egg-u, llwyed o reolaeth I ddeall yn union sut mae rheoli'n wyau, dwi am i ni edrych ar wyau trwy lygaid cyfarwydd. I wneud hyn, rydym am edrych ffefryn i bawb: wyau 'di sgramblo. | Today, we'll really crack open our eggs, to see exactly what's going on beneath that fragile shell of theirs. |
| Braster Pam dadlau; 'llaeth, yntau fenyn?' mewn ŵy 'di sgramblo? Mae gymaint o amrywiaeth rhwng yr holl ryseitiau perffaith ŵy 'di sgramblo mewn Google search, waeth i chi chwilio am yr un gwaethaf ddim! | Fat Why 'milk, or butter?' in scrambled eggs. It's a debate that's been raging for decades. Look for the perfect scrambled egg recipe, and you'll find no more agreement between them than you would the worst recipe. |
| Ond un peth cyffredin welwch chi, sy'n gwahaniaethu'r goreuon o'r gwaethaf, fydd braster. Pam felly? Pa bŵer sydd gan fraster dros yr ŵy? Mae'r ateb, fel y disgwylir, i gyd yn y protein. | One thing you will notice, however, is that the best scrambled eggs, will always contain fat. Why? What magical property does fat possess that produces such an improvements? It's all in the protein. |
| Mi rydym ni'n gwybod yn barod bod wyau, wrth eu cynhesu, yn ceulo, wrth i broteinau tu mewn iddynt dad ac ailymrwymo. Ond, os ddaliwn i gynhesu, dal i dynhau gwna'r proteinau. Mae'r wyau'n torri, mewn geiriau eraill. | We already know that when eggs are heated, protein inside will begin to unravel and bond, leading to coagulation. However, as we continue heating, beyond coagulation, the proteins tighten, and the eggs split, into big, chewy curds. |
|
|
| Proteinau ŵy yn mynd o anrhefn (chwith) i drefn, wedi' bondio'n glos (dde) wrth eu cynhesu. | Egg proteins go from disorganised and loose (left) to organised and tightly bound (right) as they're heated. |
| Tebygolrwydd yw gwres yn y bôn. Yr uchaf yw'r gwres ar ein ŵy y mwyaf yw'r siawns bod dau brotein ynddo yn cyfarfod a'n bondio. Tra bo hyn yn digwydd, mae dŵr yn yr ŵy yn prysur droi i stem, sy'n arwain at wyau ysgafn, ffluffy. Er hynny, bydd rywfaint o ddŵr yn aros ynghlwm i'r ŵy, yn sownd yn y clymau proteinau sy'n ffurfio. Dyma le ddaw gwead tendar wyau da 'di sgramblo, a lle ddaw'r gwead rwber dyfrllyd 'na o mewn wyau gwael, lle mae gor-gwcio wedi arwain at broteinau tyn sy'n gwthio dŵr allan o'u meinwe. Sut felly, mae braster am sortio hyn i gyd? | Heating increases the chance of a chemical bond forming between two proteins. Whilst this is happening, water in the eggs is turning to steam, contributing to the 'fluffiness' that we look for. Meanwhile, some moisture is retained, lending a tenderness to the cooked eggs. However, as cooking continues and more bonds form, the protein network tightens, and water is forced out, leading to the a dry, overcooked texture. So what's fat to do with all this? |
|
|
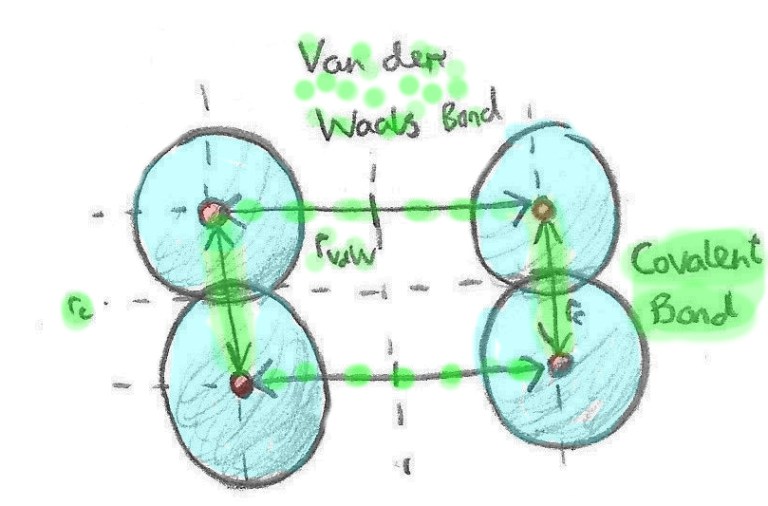
| Blanced i brotein, dynna yw braster. Er bod bondio protein-protein llawer cryfach (bondiau cofalent), mi wneith braster fondio i brotein. Mi wna hyn drwy rymoedd gwan rhyngfolecylaidd (Van der Waals) sy'n deillio o anghyfartaledd rhwng gwefrau molecylau. Be olygai hyn felly yw bod braster yn gwneud hi'n anodd iawn i brotein fondio i'w gilydd yn gryf, heb fod molecylau braster yn eu ffordd. Y canlyniad yw bod y proteinau'n bondio'n arafach felly'n dal dŵr yn hirach ac felly'n cynhyrchu'r wyau fluffy 'da ni ar eu hol. | Fat, as it turns out, coats protein. Although not quite as strong as protein-protein (covalent) bonds, fat and protein will loosely bond via inter-molecular (Van der Waals) forces from charge imbalances. The result is protein-protein bonds won't form as readily in the presence of fat. This slows down the bonding process, leading to greater water retention between protein, resulting in fluffier eggs. |
|
|
| Protein wyau'n cael eu cynhesu efo (chwith) a heb (dde) fraster. Fel y gwelwn, mae braster yn lleihau colledion dŵr drwy rwystro rhyngweithio rhwng protein. | Egg proteins heated with (left) and without (right) fat. Fat reduces water loss by inhibiting protein interaction. |
| Dyma pam bo' ryseitiau da wyau 'di sgramblo yn gofyn am riw ffurf o laeth neu fenyn. Ond mae dal terfyn i allu braster, a faint fedrwn i ychwanegu. Er enghraifft, mi wneith ychwanegu llefrith gynyddu’r dŵr yn ein hwyau, yn o gystal â'r braster. Mae dŵr yn medru dal lot o wres felly wrth gyflwyno mwy o ddŵr, mi rydym ni'n cynyddu’r amser gymerith hi i'r wyau gwcio (angen mwy o wres). Ar yr un llaw, mae hyn yn beth da i wyau 'di sgramblo, gan ei fod o'n debygol o gynhyrchu mwy o stem wrth bobi, sydd yn arwain at wyau mwy ysgafn. Ar y llaw arall, os oes angen i'r wyau gwcio'n o handi, mi fedd mwy o ddŵr yn niweidio ansawdd yr wyau. Er engrafiff, os ydw i'n paratoi omlet, mi fydd y cynnydd mewn amser cwcio yn arwain at omlet sydd mwy fel gwadan esgid na brecwast soffistigedig. | This is why dairy added to eggs produces tender, fluffier cooked eggs. But there is a limit to how much we can add. Adding milk, for example, increases the water content of the eggs, thereby significantly increase their heat capacity and hence, cooking time. On the one hand, this is good for scrambled eggs as it will generate more steam and have more moisture to retain, however, for an omelet, which needs to be more compact and foldable, the added liquid will prolong the cooking time, toughening the omelet. |
| Dyma pam mae ryseitiau omlet yn aml yn awgrymu ychwanegu menyn i'r badell, ac nid at yr wyau. Gan fod cynnwys braster menyn llawer uwch na llefrith (cyfrannedd o tua 4:1, braster i ddŵr, lle bo' llefrith yn agosach at 1:30!). Unwaith mae'r wyau'n cael eu hychwanegu i'r badell, mae'r menyn tawdd yn gweithio ei ffordd drwy'r gymysgedd ŵy, yn gorchuddio'r proteinau heb gynyddu’r amser cwcio gormod. (Ffordd hyd yn oed mwy slei o wneud hyn yw ychwanegu menyn 'di rewi i'r badell. Y syniad yw bo o'n arafu cwcio jest digon iddo allu lledu trwy'r ŵy yn fwy trylwyr na menyn sy'n boeth yn barod). | This is why omelet recipes will more often ask for you to butter the pan, instead of the eggs. Butter has a much higher fat to water ratio than milk (around 4:1, as opposed 1:30!). Once eggs are added to a buttered pan, the butter disperses throughout the egg, coating the protein in fat and producing a tender omlet, without significantly increasing its cooking time. (An even craftier trick is to use frozen butter in the pan as it will melt slowly and disperse more evenly throughout the eggs as they cook). |
| Halen Tarddbwynt bob chwedl coginio sy'n mynd. Cewch chi ddim un gegin, heblaw am un ateb sy'n bendant ar y mater o halltu wyau. Tra bo un cogydd yn halltu bob cam o'r ffordd, mi wneith un arall ddal bob gronyn yn ôl nes bo'r ŵy yn barod i'w blatio. Beth felly ydym ni i wneud efo'r holl straeon 'ma? Ymchwilio, wrth gwrs! | Salt The greatest myth maker of them all. Whilst some chefs salt all their eggs, others won't let a grain near them until the dish is seconds from service. What are we to make of all this then? The answer, as always, is in the science. |
| Os edrychwn i'n ôl i'n dyddiau TGAU Cemeg, mi gofiwn i fod halen yn sylwedd cyfansawdd ïonig. Golygai hyn bod yr atomau sy'n eu cyfansoddi yn atynnu ei gilydd drwy rym electrostatig rhwng ionau â gwefrau gwrthwynebol. Yn achos halen, anion negatif Clorin sy'n rhannu bond ïonig efo catïon positif Sodiwm. | Salt, as we all fondly recall, is defined as an ionic compound formed of two charged ions, a negative anion, and positive cation. In the case of table salt, we have a Sodium cation sharing an ionic bond with a Chlorine anion. |
|
|
| Sodium yn bondio i Clorin wrth drosglwyddo electron iddo, i ffurdio dau ion positif a negatif | Sodium atom bonds to Chlorine by donating an electron, forming positive and negative ions |
| Mae'r ionau yna’n medru effeithio'r wyau mewn dwy ffordd. Trwy rymoedd rhyngfoleciwlaidd (grym rhwng proteinau) a mewnfoleciwlaidd (o fewn proteinau). | These ions effect egg proteins in two ways, influencing the intermolecular (force between) and intramolecular (force within) interaction between proteins. |
| Edrychwn ni gyntaf ar y grymoedd mewnfoleciwlaidd. Wrth ychwanegu halen at wyau, y peth cyntaf ddigwyddith yw bod yr halen yn hydoddi. Hynny yw, mi rannith y crisialau halen lawr i'w ionau cyfansawdd, Sodiwm a Chlorin. Wedi hydoddi, mi symudith yr ionau tuag at ranbarthau o wefrau anghytbwys. Gan fod y gwefrau rhanbarthol 'ma'n rhemp o fewn, ac ar hyd proteinau, mi wneith halen achosi i broteinau ddatod yn haws, drwy gydbwyso'r gwefrau'r sy'n eu dal at ei gilydd. | Firstly, let's look at the intramolecular forces. When we add table salt to uncooked egg, it dissolves, splitting into it's constituent Sodium and Cloride ions. These ions will then seek out and neutralise regions of opposing charge, which occur all along the length of the egg proteins. With charge imbalances neutralised, sections of the protein will no longer attract one another, allowing the protein to unfurl. |
|
|
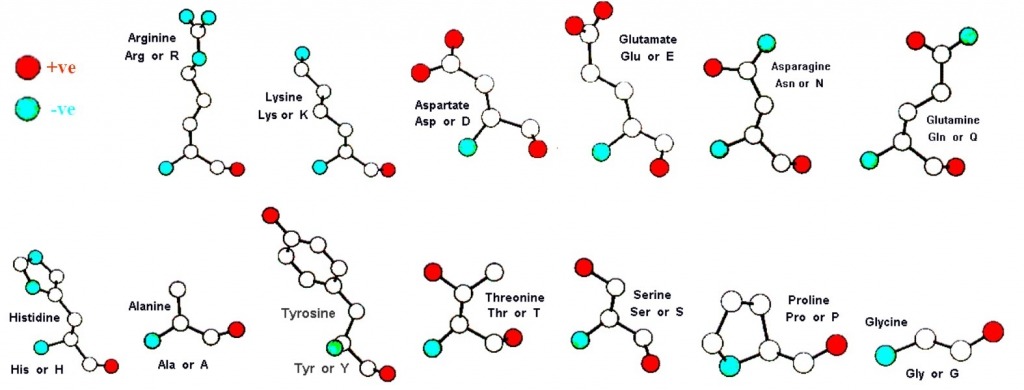
| Strwythur yr 20 asid amino a lleoliad eu gwefrau | The charge configurations of the 20 amino acids |
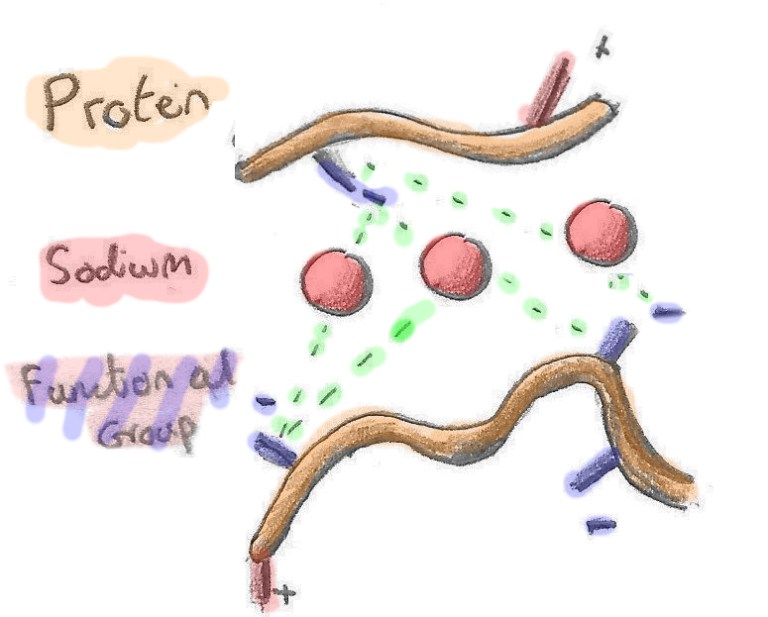
| Yr ail effaith mae halen yn ei gael wrth gwrs, yw'r effaith rhyngfoleciwlaidd. Tueddu tuag ato fo'n gyfan gwbl negatif yw proteinau ŵy felly mi fydd y catïon Sodiwm (gwefr bositif) yn tueddu i gasglu o'u cwmpas. Mi wneith hyn, yn ei dro, achosi i fwy o broteinau gasglu o'u cwmpas nhw ac felly ar y cyfan, mi fydd angen llai o egni i'r holl beth geulo. Golygai hyn y bydd wyau 'di halltu'n fwy parod i geulo, ac ar dymheredd is na rhai heb eu halltu. Catalydd felly yw halen ar gyfer i ryngweithio proteinau. | Secondly, Sodium cations will attract the overall negative egg proteins, which converge around them. Easier convergence means greater intermolecular interaction between proteins, meaning salted eggs coagulate more readily, and at a lower temperature, than unsalted eggs. Salt therefore behaves as a catalyst for protein interaction, lowering the energy required for two proteins to interact. |
|
|
| Ond mae gan halen dric bach arall buddiol. Er bod yr ionau'n tynnu proteinau at ei gilydd, mae dal angen lle arnynt. Be sy'n digwydd felly yw bo mas ffisegol yr ionau'n yn rhwystro'r proteinau rhag casglu'n rhy agos at ei gilydd. Bydd hyn yn arbed y rhwydwaith proteinau rhag bondio'n rhy dyn, sydd felly'n gwneud hi'n anoddach gor gwcio'r wyau. Felly mae halen yn cadw'ch wyau'n ysgafn a hufennog am hirach. | However, it also makes overcooking more difficult as the ions that bring the proteins together also act as a buffer, keeping them from getting too close. This inhibits the protein network from becoming too tight, so your eggs stay creamier and fluffier for longer. |
| Dyna i chi eich ateb felly yn de! Gwyddoniaeth (go drwm) o'r diwedd wedi rhoi Wil i'w wely! Mi ddylech chi fo'n halltu eich wyau cyn pobi er mwyn macsimeiddio'r dŵr sy'n cael ei ddal ac i finimeiddio'r amser pobi sydd ei angen i gael wyau tendar ac ysgafn. | So there's your answer! Science (and some pretty heavy science at that) has answered our prayers! You should be salting your eggs before cooking to help maximize water retention and minimize cooking time for even more tender and fluffy results. |
| Finegr, Sudd Lemon a sylweddau asidig eraill Os mae natur ïonig halen sy'n arwain at yr holl ddadlau ma’, pam felly bod asid yn cael ei absenoli o'r ddadl? Cyrd Lemon yw'r ateb i hynnu. | Vinegar, Lemon Juice and other acids Another culinary quirk of electrostatics for you here. If salt is such a controversial topic that's all about the ions, why isn't acid on the top 10 kitchen controversies scale? Lemon curd is all I can say to that. |
| Diolch i On Food and Cooking, a rysáit wyau 'di sgramblo gan Le Patissier françois, mae'n bur debyg mae dechrau bywyd fel wyau 'di sgramblo gwnaeth y cyrd lemon cyntaf. Yn y rysáit, mae o'n galw am ychwanegu sudd grawnwin 'di chwerwi at wyau melys, wrth ychwanegi y byddai sudd lemon neu oren hefyd yn opsiwn. Beth sy'n gyffredin am y tri sudd yma yw eu bod nhw i gyd yn cynnwys asid. Mae sudd grawnwin, gydag amser, yn datblygu asid ethanoig drwy ocsidiad ethanol (sydd ei hun yn gynnyrch o eplesu glwcos a ffrwctos gan furum naturiol ar groen y grawnwin) ac mae sudd lemon a sudd oren yn cynnwys asid sitrig naturiol. | Courtesy of On Food and Cooking, and a scrambled egg recipe from Le Patissier françois, it turns out that lemon curd started life as a sweetened scrambled egg recipe that used sour grape juice instead of milk, and which stated that lemon or orange juice could also be used. What these three juices have in common is that they all contain acid. Sour grapes contain ethanoic acid from the oxidization of ethanol (which is its self a by product of glucose and fructose fermentation performed by wild yeast present on the grape's skin) and both lemons and oranges naturally contain citric acid. |
|
|
| Ion Hydrogen, proton rhydd: yr hyn sy'n gwneud asid yn asidig | What makes acid acidic: hydrogen ions |
| Yn fras, sylwedd sy'n cynnwys atomau Hydrogen yw asid. Atomau Hydrogen wedi colli electron i fod yn fanwl gywir, sy'n gwneud asid yn gawl o brotonau rhydd. | Acid, at its most fundamental, is a liquid containing Hydrogen atoms. Hydrogen atoms that all have their electrons missing in fact, which means they're just protons, floating units of positive charge. |
| Yn union fel ionau Sodiwm mewn halen, mae ychwanegu asid at wyau'n cyflwyno llu o wefrau positif i'r proteinau ŵy. Y cryfaf yw'r asid, yr uchaf yw crynodebau'r ionau. Wedi'u hychwanegu, mi geith yr ionau Hydrogen yr union un effaith ar y proteinau a'r halen, yn lleihau'r tymheredd ceulo sy'n arbed gor gwcio. | Just like liberating those free Sodium cations from salt, adding an acid to eggs introduces a slew of positive charges to the egg proteins. The stronger the acid, the higher the concentration of ions. The Hydrogen ions then act like the salt, lowering the coagulation temperature, preventing overcooking. |
| Dyma sut mae cyrd lemon yn ffurfio. Efo crynodebau mor uchel o ddŵr ac ionau Hydrogen, mi wrthodith yr wyau geulo'n gyfan gwbl. Yn lle, mi wneith y gymysgedd ffurfio gel, a dyma ydym ni'n adnabod fel cyrd. | This is how lemon curd forms. With high concentration of water and Hydrogen ions present, heated eggs will simply refuse to bond fully. Instead, they'll form a gel that we refer to as a curd. |
| Siwgwr Gymaint a ydym ni wrth ein boddau efo siwgwr ym myd pobi, amhurdeb yn unig yw siwgwr mewn wyau. Maint ffisegol y molecylau siwgr sy'n rhwystro proteinau rhag rhyngweithio. Mae ychwanegu siwgwr at wyau felly'n cynyddu’r tymheredd ceulo a'n arbed yr wyau rhag malu (fel y gwelwn mewn cacen) | Sugar As revered as it is in the baking world, sugar in eggs behaves very similar to fat, as an impurity. The presence of large sugar molecules physically getting in the way of the proteins serves to inhibit protein interaction. This then means that adding sugar to eggs will increase their coagulation temperature, inhibiting curdling and overcooking. |
| Ond, beth sy'n ddiddorol yw'r ffaith bod siwgwr yn rhwystro proteinau ŵy rhag datod yn y lle cyntaf, yn y camau cyntaf o gynhesu. Wrth ryngweithio efo'r clympiau protein, mae molecylau siwgwr yn cynyddu'r grym mewnfoleciwlaidd sy'n gwneud hi'n anoddach ceulo wyau melys. | Interestingly however, sugar also inhibits egg proteins from initially unfolding by increasing the intramolecular forces binding proteins to themselves. This makes it even more difficult to curdle eggs with sugar added to them. |
| Peth arall mae siwgwr yn wneud sy'n unigryw iddo yw dal dŵr. Molecylau pegynol yw rhai siwgwr (neu swcros i fod yn dechnegol), sy'n golygu bod gan ei folecylau un pen positif a phen arall negatif. Mae molecylau dŵr hefyd, yn begynol, ac felly mae grym atynnol cryf rhwng pegynau molecylau dŵr a siwgr. | One thing that sugar does do better than anything else however is attract water. Sugar (or sucrose) is a polar molecule, meaning it has both a positive and a negative charge. Sugar also strongly attracts water, another highly polar molecule, making sugar hygroscopic, water attracting. |
| Dyma pam bod siwgwr mor hanfodol i chwipio meringue, o natur hygrosgopig y siwgwr. Erthygl arall, i ddiwrnod arall yn fanno! | This lends its self well when making a meringue due to the hygroscopic properties of sugar, which prevents egg foams from leaking. An article topic unto its self, and maybe, for another day! |
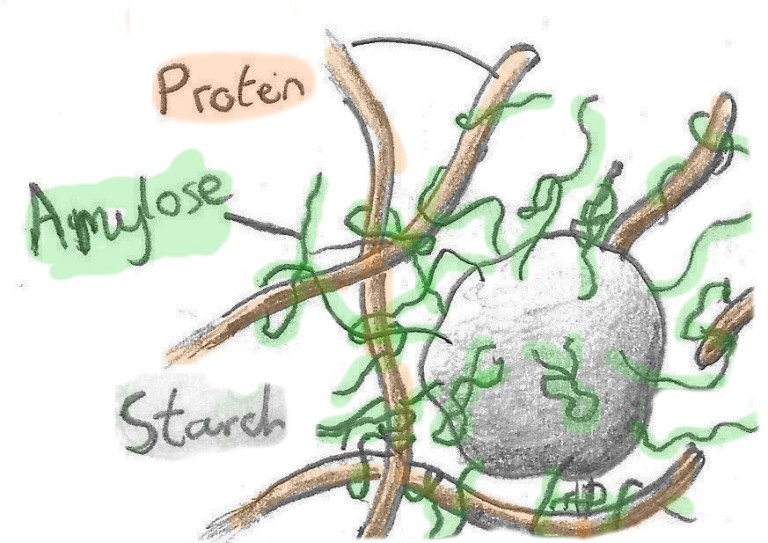
| Starts Mae starts, fel braster a siwgwr, yn ymddwyn fel amhurdeb, yn gwanhau'r crynodeb protein sy'n arwain at dymheredd ceulo uwch. Ond efallai bod hyn yn symleiddio gormod. | Starch Starch, like fat and sugar, bulks up the egg mixture, diluting the protein concentration thereby increasing egg coagulation temperature. |
| Tua dwywaith trymach na swcros, mae starts (amylos ac amylopectin) yn ddigon mawr i lapio ei hun i mewn i ronyn sffêr garw. Fel bownsars y byd bwyd, mae presenoldeb yn unig yn arbed bob math o ryngweithio cemegol. | Starches are particularly effective due to their sheer size. They're large, spherical granules, that, much like a food science bouncer, will simple block interactions from occurring. |
|
|
| Ond mae pethau diddorol yn digwydd wrth i ni eu cynhesu. Wrth i'r starts gynhyrfu, mae'r gronyn yn dechrau dadfeilio, ac mae molecylau llai, fel amylos, yn dechrau ymestyn ohono. Wrth i'r tentaclau siwgwr yma ledaenu, mi gymerant nhw afael yn y proteinau o'u cwmpas, yn ei gwneud hi'n anoddach byth i'r proteinau rwydweithio. | One interesting thing about starch however is that when heated, they'll release some of their own trapped molecules. One such molecule is amylose, a long chain sugar that's released from the starch granule and much like a tentacle, will coat the egg proteins to make it even more difficult for a protein network to form. |
| Dyma pam bod hi'n bosib pobi cwstard i 82°C cyn bod o'n hyd yn oed dechrau twchu, lle bod wyau ben ei hunain yn ceulo ar 65°C. Dim bod hyn yn gwneud y broses pobi'n haws. Yr unig beth mae'r starts yn gwneud yw codi'r pwynt ceulo i dymheredd llawer anoddach i'w reoli. Dyma pam bod creu cwstard ŵy esmwyth yn gymaint o gamp, a pam bod angen bod mor ofalu yn pobi cwstard, fel crème brûlée. | This is why egg custards can be heated to 82°C before thickening whilst eggs will coagulate around 65°C. This doesn't make it any easier however as all you've done is increased the temperature at which the custard will curdle to a higher, less manageable temperature. This is one of the reasons why being able to make a smooth egg custard is such a valued skill, and why baked custard such as crème brûlée are so delicate. |
| Mae o hyd mwy i'w ddysgu... Felly dyna i chi, yn union sut mae deall yr ŵy 'di sgramblo perffaith. Os ydych chi'n ysu i ymarfer yr hyn ydych chi newydd ddysgu, 'dwi'n eich herio i drio ein rysáit Cyrd Lemon, ac i ddweud wrthym ni sut hwyl gafoch chi yn y comments! | There's always more to learn... So there you have it, the answer to the perfect scrambled egg! If your feeling adventurous though, you can tackle our Lemon Curd recipe and let know how you get on in the comment. But for now go out and utilize your newfound egg theory. Brunch domination is now firmly in your grasp. |
artisaniaeth.com / youtube.com/channel/UC0mEoGDw7GKMta-OAQNecLA